Selective adsorption of oxidized yeast glucan for Pb2+: Influencing factors, adsorption site and mechanism
Shuai Chen, Yujie Guo, Wudan Cai, Qilin Huang
Highlights
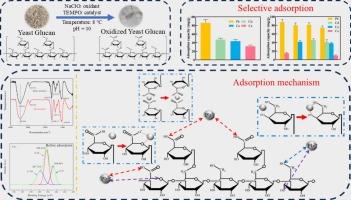
- Oxidized yeast glucan (OYG) selectively adsorbed Pb2+ from quaternary ion solution.
- OYG with high oxidation degree exhibited a greater adsorption capacity of Pb2+.
- OYG had the maximum adsorption capacity for Pb2+ at pH 6–7 and a dose of 20 mg.
- Adsorption is a spontaneous endothermic process and inherently heterogeneous.
- Adsorption is from electrostatic force between hydroxyl and carboxyl of OYG and Pb2+.
Abstract
This work investigated the influencing factors and mechanism of Pb2+ adsorbed by oxidized yeast glucan (OYG). Firstly, OYG showed greater Pb2+ adsorption performance than yeast glucan, and the adsorption capacity was greater with the oxidation degree increased. The optimal pH range for Pb2+ adsorption was 6–7. The adsorption capacity of Pb2+ reached its maximum of 86.68 and 96.88 mg/g for 20 mg of OYG1 and OYG2, respectively. In addition, OYG exhibited selective adsorption, with adsorption capacity in the order: Pb2+ > Cd2+ > Cu2+ > Ca2+. Then adsorption isotherm was established by fitting Langmuir and Freundlich isotherm adsorption models. Freundlich model could better fit the adsorption process of lead by OYG, indicating that the adsorption process was heterogeneous. According to the Langmuir model, the maximum lead adsorption capacities of OYG1 and OYG2 at 298 K were 100.70 and 131.06 mg/g, respectively. Thermodynamic data indicated a spontaneous endothermic process (ΔG < 0, ΔH > 0), accompanied by increased system disorder (ΔS > 0), involving physical and chemical adsorptions. Finally, FT-IR and XPS results revealed -COOH and -OH were the primary adsorption sites in OYG, and the interaction between these groups and Pb2+ was mainly achieved by electrostatic interaction (or ion-dipole interaction).
https://www.sciencedirect.com/science/article/abs/pii/S0141813024078838

Comments