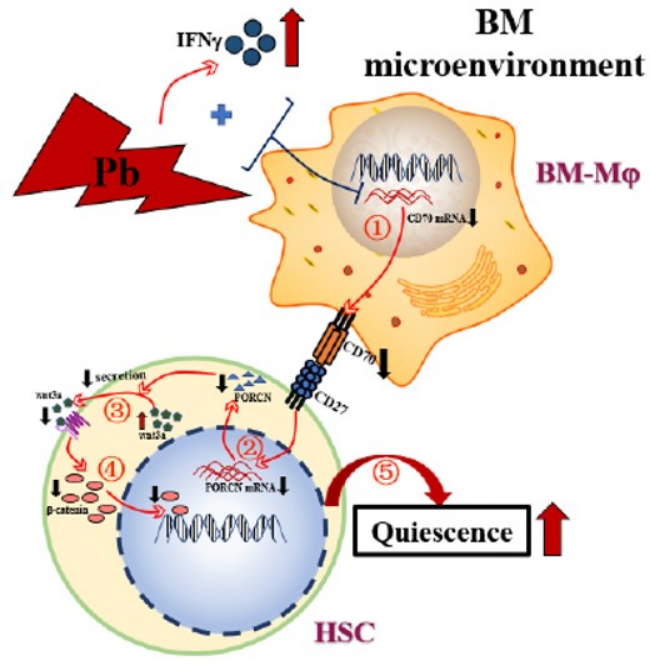
In conclusion, as summarized in Figure 9, an occupationally relevant level of Pb exposure induces IFNγ production in the BM, and thereafter a synergistic action of Pb and IFNγ on BM-Mφ decreases their expression of CD70; the weakened CD70 signaling from BM-Mφ leads to suppressed expression of PORCN in HSC, which impedes the transportation of Wnt3a from the cytoplasm to the cell membrane to impair the β-catenin signaling in HSC and thereby increases the quiescence of HSC.
Lead exposure suppresses the Wnt3a/β-catenin signaling to increase the quiescence of hematopoietic stem cells via reducing the expression of CD70 on bone marrow-resident macrophages
Toxicological Sciences, Volume 195, Issue 1, September 2023, Pages 123–142, https://doi.org/10.1093/toxsci/kfad067
Published:
12 July 2023
Abstract
Lead (Pb) is a heavy metal highly toxic to human health in the environment. The aim of this study was to investigate the mechanism of Pb impact on the quiescence of hematopoietic stem cells (HSC). WT C57BL/6 (B6) mice treated with 1250 ppm Pb via drinking water for 8 weeks had increased the quiescence of HSC in the bone marrow (BM), which was caused by the suppressed activation of the Wnt3a/β-catenin signaling. Mechanically, a synergistic action of Pb and IFNγ on BM-resident macrophages (BM-Mφ) reduced their surface expression of CD70, which thereby dampened the Wnt3a/β-catenin signaling to suppress the proliferation of HSC in mice. In addition, a joint action of Pb and IFNγ also suppressed the expression of CD70 on human Mφ to impair the Wnt3a/β-catenin signaling and reduce the proliferation of human HSC purified from umbilical cord blood of healthy donors. Moreover, correlation analyses showed that the blood Pb concentration was or tended to be positively associated with the quiescence of HSC, and was or tended to be negatively associated with the activation of the Wnt3a/β-catenin signaling in HSC in human subjects occupationally exposed to Pb. Collectively, these data indicate that an occupationally relevant level of Pb exposure suppresses the Wnt3a/β-catenin signaling to increase the quiescence of HSC via reducing the expression of CD70 on BM-Mφ in both mice and humans.
lead, quiescence, HSC, Wnt3a/β-catenin, BM-Mφ, CD70, PORCN
Topic:
- signal transduction
- hematopoietic stem cells
- internship and residency
- macrophages
- mice
- medical residencies
- cell cycle quiescence
Issue Section:
Lead (Pb) is a heavy metal that broadly exists in the environment (Frank et al., 2019; Han et al., 2018; Lermen et al., 2021). Pb enters human bodies and accumulates in tissues mainly through the consumption of food and water and occupational exposure (Ahmad et al., 2018; Khan et al., 2015; Mohammadyan et al., 2019; Wu et al., 2023). Pb is toxic to the immune system, and one of the characteristics is that Pb skews toward a humoral immune response at the expense of a cellular immune response (Gao et al., 2007; Kishikawa et al., 1997; Mishra, 2009).
Hematopoietic stem cells (HSC) are able to self-renew and differentiate into all kinds of blood cells to replenish the blood cell pool (Eaves, 2015). In mammals, most HSC reside in a micro-environment named niche in the bone marrow (BM), which is crucial for maintaining the function of HSC (Pinho and Frenette, 2019). In steady state, most HSC are quiescent (in G0 stage), and only a small part of HSC proliferates to self-renew and differentiate into progenitor cells (Passegue et al., 2005; Weissman and Shizuru, 2008). The cell cycle of HSC is tightly orchestrated by both extrinsic factors and intrinsic networks in the BM, in order to fulfil hematopoietic demand and minimize the potential replication-associated stress (Nakamura-Ishizu et al., 2014; Singh et al., 2020). Although quiescence has been widely recognized as a protective mechanism for maintaining HSC integrity and preventing HSC from exhaustion, enhanced quiescence of HSC may also bring about deleterious outcomes for human beings. For example, increased quiescence of HSC may result in a decreased number of blood cells to cause or exacerbate anemia (Luzzatto and Risitano, 2018). Moreover, increased quiescence of HSC may lead to less production of immune cells and thereby compromise the immune responses against infections (Howard et al., 2021).
The canonical Wnt signaling is characterized by the activation of β-catenin in the presence of Wnt ligands (Clevers, 2006; Staal and Clevers, 2005), which has been implicated in promoting the proliferation of HSC (Reya et al., 2003; Zhao et al., 2007). The synthesized Wnt proteins are acetylated by porcupine O-acyltransferase (PORCN) in the endoplasmic reticulum (ER), which facilitates the transportation of the Wnt proteins through the Golgi apparatus to the extracellular environment (Barrott et al., 2011; Hofmann, 2000; MacDonald et al., 2009; Willert et al., 2003). Activation of the canonical Wnt signaling is initiated by the binding of the Wnt proteins to their specific surface receptors Frizzled and LRP, which subsequently results in the phosphorylation of glycogen synthase kinase (GSK)3β (MacDonald et al., 2009). The phosphorylation of GSK3β reduces the phosphorylation of β-catenin, which decreases the degradation of β-catenin and thereby increases the accumulation of β-catenin in cytoplasm and nucleus (Ha et al., 2004; MacDonald et al., 2009). HSC can receive the Wnt signaling in an autocrine or paracrine manner (Luis et al., 2009; Reya et al., 2003; Reya and Clevers, 2005; Routledge and Scholpp, 2019). Indeed, the Wnt3a/β-catenin signaling has been shown to promote the proliferation of HSC in an autocrine manner (Luis et al., 2009; Reya et al., 2003). Interestingly, the activation of the Wnt/β-catenin signaling in leukemia cells was critically dependent on the CD70 signaling (Riether et al., 2017; Schurch et al., 2012).
The BM-resident macrophages (BM-Mφ) are tissue-specific phagocytes that can regulate the function of HSC (McCabe and MacNamara, 2016; Seyfried et al., 2020). BM-Mφ are not only able to maintain the HSC pool in the BM or mobilize the HSC to the periphery but also can drive or suppress the proliferation of HSC, depending on the context of differential environmental stimuli (Christopher et al., 2011; McCabe et al., 2015; Winkler et al., 2010). Regarding the mechanism of the impact of BM-Mφ on HSC, although still largely unknown, it is suggested that BM-Mφ may influence HSC via a direct cellular contact between BM-Mφ and HSC, or through secreting soluble factors (Hur et al., 2016; Ludin et al., 2012).
Previously, we and others have reported that Pb can impact the function of hematopoietic stem and progenitor cells in the BM (Gao et al., 2007; Li et al., 2017; Liu et al., 2015; Lutton et al., 1984; Zhao et al., 2021; Zhu et al., 2020). Regarding the impact of Pb on HSC, we have found that an occupationally relevant level of Pb exposure, but not an environmentally relevant level of Pb exposure, increases the quiescence of HSC via a synergistic action of Pb and IFNγ on BM-Mφ, but not through a direct action of Pb on HSC (Zhao et al., 2021). Pb is known to cause anemia, which has been widely recognized as a consequence of a direct action of Pb on mature blood cells (Baranowska-Bosiacka et al., 2009). Interestingly, we found that the increased quiescence of HSC by Pb exposure may contribute to anemia (Zhao et al., 2021), thus providing a new mechanistical angle for Pb-caused anemia. Therefore, unveiling the mechanism for Pb-increased quiescence of HSC is important for conceptually advancing the understanding for Pb toxicities. As Pb broadly exists in the environment, understanding the mechanism of Pb impact on HSC is significant for public health. The aim of this study was to investigate the mechanism of the increased quiescence of HSC caused by Pb exposure. We hypothesized that the BM-Mφ might increase the quiescence of HSC via regulating the canonical Wnt signaling in HSC during Pb exposure. We used mouse models as well as human samples to test our hypothesis.

Comments2
The truth we can't handle: Why Blood Cancer Immune System Stem
The truth we can't handle: Why Blood Cancer Immune System Stem Cell Genocide Blood study tracks what happens to stem cells decades after a transplant "research highlights that age is more than just a number"
https://icesaturn.com/StemCells
My best self-diagnosis to date.
My best self-diagnosis to date.